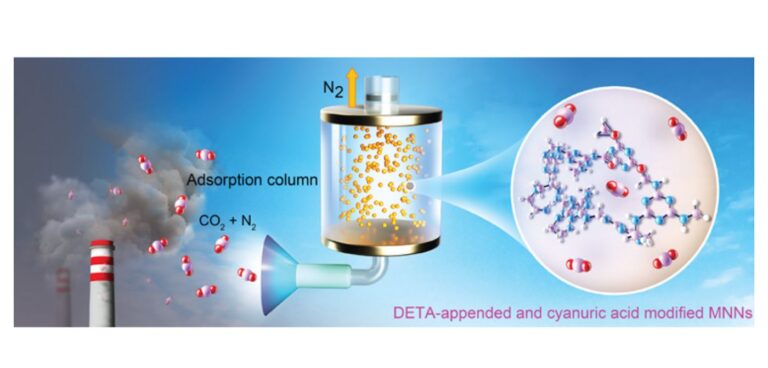
Researchers at UC Berkley in the USA have created a cheap and energy efficient means of carbon capture using the inexpensive polymer melamine — the main component of Formica. The process has the potential to be applied to either large scale emissions sources such as smokestacks or scaled down for automotive use.
According to the research, the new material is simple to make, requiring primarily off-the-shelf melamine powder — which costs about US$40 per ton — along with formaldehyde and cyanuric acid, a chemical that, among other uses, is added with chlorine to swimming pools.
“We wanted to think about a carbon capture material that was derived from sources that were really cheap and easy to get. And so, we decided to start with melamine,” said Jeffrey Reimer, professor of the Graduate School in the Department of Chemical and Biomolecular Engineering at the University of California, Berkeley, and one of the corresponding authors of the paper.
The so-called melamine porous network captures carbon dioxide with an efficiency comparable to early results for another relatively recent material for carbon capture, metal organic frameworks, or MOFs. UC Berkeley chemists created the first such carbon-capture MOF in 2015, and subsequent versions have proved even more efficient at removing carbon dioxide from flue gases, such as those from a coal-fired power plant.
Haiyan Mao, a UC Berkeley postdoctoral fellow who is first author of the paper, said that melamine-based materials use much cheaper ingredients, are easier to make and are more energy efficient than most MOFs. The low cost of porous melamine means that the material could be deployed widely.
“In this study, we focused on cheaper material design for capture and storage and elucidating the interaction mechanism between CO2 and the material,” Mao said. “This work creates a general industrialization method toward sustainable CO2 capture using porous networks. We hope we can design a future attachment for capturing car exhaust gas, or maybe an attachment to a building or even a coating on the surface of furniture.”
Notably, the melamine porous network with DETA and cyanuric acid modification captures CO2 at about 40°C, slightly above room temperature, and releases it at 80°C, below the boiling point of water. These are much lower temperatures than required by other capture methods and represent considerable energy savings.
The work is a collaboration among a group at UC Berkeley led by Reimer; a group at Stanford University led by Yi Cui, who is director of the Precourt Institute for Energy, the Somorjai Visiting Miller Professor at UC Berkeley, and a former UC Berkeley postdoctoral fellow; UC Berkeley Professor of the Graduate School Alexander Pines; and a group at Texas A&M University led by Hong-Cai Zhou. Jing Tang, a postdoctoral fellow at Stanford and the Stanford Linear Accelerator Center and a visiting scholar at UC Berkeley, is co-first author with Mao. Reimer is also a faculty scientist at Lawrence Berkeley National Laboratory.
In its research, the Berkeley/Stanford/Texas team focused on the common polymer melamine, which is used not only in Formica but also inexpensive dinnerware and utensils, industrial coatings and other plastics. Treating melamine powder with formaldehyde — which the researchers did in kilogram quantities — creates nanoscale pores in the melamine that the researchers thought would absorb CO2.
Mao said that tests confirmed that formaldehyde-treated melamine-adsorbed CO2 somewhat, but adsorption could be much improved by adding another amine-containing chemical, DETA (diethylenetriamine), to bind CO2. She and her colleagues subsequently found that adding cyanuric acid during the polymerization reaction increased the pore size dramatically and radically improved CO2 capture efficiency: Nearly all the carbon dioxide in a simulated flue gas mixture was absorbed within about 3 minutes.
Mao and her colleagues conducted solid-state nuclear magnetic resonance (NMR) studies to understand how cyanuric acid and DETA interacted to make carbon capture so efficient. The studies showed that cyanuric acid forms strong hydrogen bonds with the melamine network that helps stabilize DETA, preventing it from leaching out of the melamine pores during repeated cycles of carbon capture and regeneration.
The Reimer and Cui groups are continuing to tweak the pore size and amine groups to improve the carbon capture efficiency of melamine porous networks, while maintaining the energy efficiency. This involves using a technique called dynamic combinatorial chemistry to vary the proportions of ingredients to achieve effective, scalable, recyclable and high-capacity CO2 capture.
The full paper can be found here.