MIT researchers have discovered how branch-like metallic filaments can sap the power of solid-state lithium batteries and also how these filaments can be diverted. The finding is expected to enable the creation of smaller, lighter and safer rechargeable lithium batteries.
According to the university, the key to this potential leap in battery technology is to replace the liquid electrolyte that sits between the positive and negative electrodes with a much thinner, lighter layer of solid ceramic material, and replace one of the electrodes with solid lithium metal. This was expected to reduce the size and weight of the battery and remove the safety risk associated with the flammable liquid electrolytes. However, that quest has been beset with one big problem – dendrites. Dendrites are projections of metal that can build up on the lithium surface and penetrate the solid electrolyte, eventually crossing from one electrode to the other and shorting out the battery cell.
The new research, published in the journal Joule in a paper by MIT Professor Yet-Ming Chiang, graduate student Cole Fincher and five others at MIT and Brown University, seems to resolve the question of what causes the harmful dendrite formation. It also shows how dendrites can be prevented from crossing through the electrolyte.
Chiang said, “In our earlier work, we made a surprising and unexpected finding, which was that the hard, solid electrolyte material used for a solid-state battery can be penetrated by lithium, which is a very soft metal, during the process of charging and discharging the battery, as ions of lithium move between the two sides.”
This ion movement causes the volume of the electrodes to change. That causes stresses in the solid electrolyte, which has to remain fully in contact with both of the electrodes that it is sandwiched between. Chiang continued, “To deposit this metal, there has to be an expansion of the volume because you’re adding new mass. So, there’s an increase in volume on the side of the cell where the lithium is being deposited. And if there are even microscopic flaws present, this will generate pressure on those flaws that can cause cracking.”
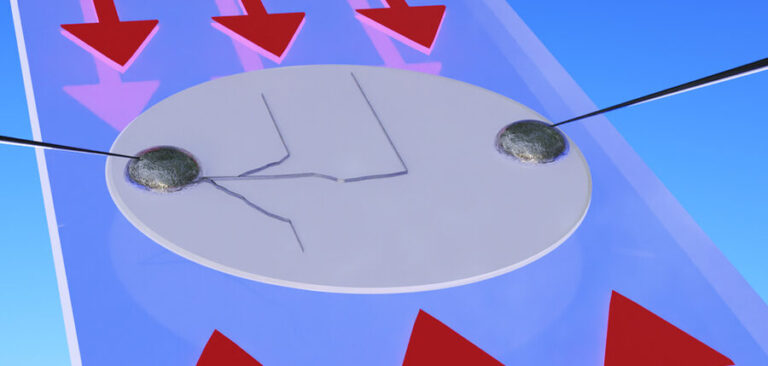
Those stresses, the team has now shown, cause the cracks that enable dendrites to form. The solution to the problem turns out to be more stress, applied in just the right direction and with the right amount of force. While previously, some researchers thought that dendrites formed by a purely electrochemical process rather than a mechanical one, the team’s experiments demonstrated that it is mechanical stresses that cause the problem.
As the process of dendrite formation normally takes place deep within the opaque materials of the battery cell and cannot be observed directly, Fincher developed a way of making thin cells using a transparent electrolyte, ensuring the whole process can be seen and recorded. He said, “You can see what happens when you put a compression on the system, and you can see whether or not the dendrites behave in a way that’s commensurate with a corrosion process or a fracture process.”
The team demonstrated that they could directly manipulate the growth of dendrites simply by applying and releasing pressure, causing the dendrites to zig and zag in perfect alignment with the direction of the force. The report emphasizes that applying mechanical stresses to the solid electrolyte doesn’t eliminate the formation of dendrites but does control the direction of their growth. This means they can be directed to remain parallel to the two electrodes and prevented from ever crossing to the other side, and thus rendered harmless.
In their tests, the researchers used pressure induced by bending the material, which was formed into a beam with a weight at one end. But they say that in practice, there could be many different ways of producing the needed stress. For example, the electrolyte could be made with two layers of material that have different amounts of thermal expansion, so that there is an inherent bending of the material, as is done in some thermostats.
Another approach would be to ‘dope’ the material with atoms that would become embedded in it, distorting it and leaving it in a permanently stressed state. This is the same method used to produce the super-hard glass used in the screens of smartphones and tablets, Chiang explained. And the amount of pressure needed is not extreme – the experiments showed that pressures of 150-200MPa were sufficient to stop the dendrites from crossing the electrolyte.
Fincher added, “The required pressure is commensurate with stresses that are commonly induced in commercial film growth processes and many other manufacturing processes, so should not be difficult to implement in practice.”
In fact, a different kind of stress, called stack pressure, is often applied to battery cells, by essentially squishing the material in the direction perpendicular to the battery’s plates – somewhat like compressing a sandwich by putting a weight on top of it. It was thought that this might help prevent the layers from separating. However, the experiments have now demonstrated that pressure in that direction actually exacerbates dendrite formation.
What is needed instead is pressure along the plane of the plates, as if the sandwich were being squeezed from the sides. Fincher commented, “What we have shown in this work is that when you apply a compressive force you can force the dendrites to travel in the direction of the compression, and if that direction is along the plane of the plates, the dendrites will never get to the other side.”
That could finally make it practical to produce batteries using solid electrolyte and metallic lithium electrodes. Not only would these pack more energy into a given volume and weight, but they would eliminate the need for liquid electrolytes, which are flammable materials.
Chiang said, “Having demonstrated the basic principles involved, the team’s next step will be to try to apply these to the creation of a functional prototype battery and then to figure out exactly what manufacturing processes would be needed to produce such batteries in quantity. Though we have filed for a patent, the researchers don’t plan to commercialize the system themselves, as there are already companies working on the development of solid-state batteries. I would say this is an understanding of failure modes in solid-state batteries that we believe the industry needs to be aware of and try to use in designing better products.”
The research team included Christos Athanasiou and Brian Sheldon at Brown University, and Colin Gilgenbach, Michael Wang and W Craig Carter at MIT. The work was supported by the US National Science Foundation, the US Department of Defense, the US Defense Advanced Research Projects Agency and the US Department of Energy.